Silky Cup : Safety, Quality and Standards
Silky Cup is Clinically Tested Menstrual Cup. Silky Cup is the safest menstrual cup in the world for feminine hygiene; Silky Cup has proven its quality and durability on all tests
We believe that all women should have a happy and healthy period experience. That is why we do everything we can to ensure our products are tested and manufactured with women’s health and comfort in mind.
The Silky Cup has been tested within International standards. ISO 10993 is a Quality Standard governed by the International Organization of Standardization in Geneva, Switzerland, and is recognized and practiced in over 160 countries around the world. ISO 10993 is the quality standard for medical devices.
Silky Cup is safe; Silky Cup has been developed especially for menstrual hygiene management. Silky Cup has been tested as per the United States Pharmacopeia (USP) and International Organization for Standardization (ISO) International standards. Silky Cup has passed the all necessary ISO 10993 tests with great success.
“Silky Cup has been tested according to ISO 10993-5 (Cytotoxicity), ISO 10993-10 (Mucosal Irritation), ISO 10993-10 (Sensitization), Directive 2011/65/EU Restriction of Hazardous Substances Directive (ROHS), USP <88> “CLASS VI” Tests Acute Systemic Toxicity, Intracutaneous Irritation and Intramuscular Implantation.” Repeated bending strain and flexing for crack growth test upto 1,50,000 cycles has been done for Silky Cup.
Clinical Studies
STUDY TITLE: SKIN SENSITIZATION STUDY OF SILKY CUP (MENSTRUAL CUP) IN GUINEA PIGS (MAXIMIZATION TEST)
RESULTS AND DISCUSSION
Clinical Signs of Toxicity and Mortality
No clinical signs of toxicity and mortality were observed in any of the animals in all the groups.
PHOTOGRAPHS OF CHALLENGE PHASE
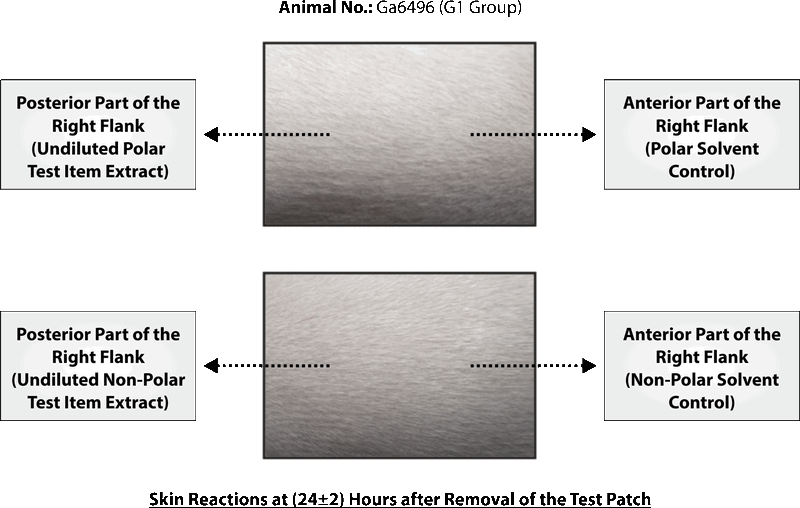
CONCLUSION
Based on the results of the experiment, it can be concluded that polar and non-polar extracts of test item Silky Cup (Menstrual Cup) was found to be “Non-sensitizer” to the skin of the Guinea pigs under the experimental conditions and doses employed as per ISO 10993-10: 2010(E).
STATEMENT OF GLP COMPLIANCE
The Study No.: BIO-TX 2110, entitled “Skin Sensitization Study of Silky Cup (Menstrual Cup) in Guinea Pigs (Maximization Test)” was performed in compliance with the OECD Principles of Good Laboratory Practice [C(97)186/Final] and in accordance with FDA Good Laboratory Practice 21 CFR Part 58.
STUDY TITLE: VAGINAL IRRITATION TEST OF SILKY CUP (MENSTRUAL CUP) IN NEW ZEALAND WHITE RABBITS
RESULTS AND DISCUSSION
Clinical Signs of Toxicity and Mortality
No treatment related clinical signs of toxicity and mortality were observed in any of the group animals.
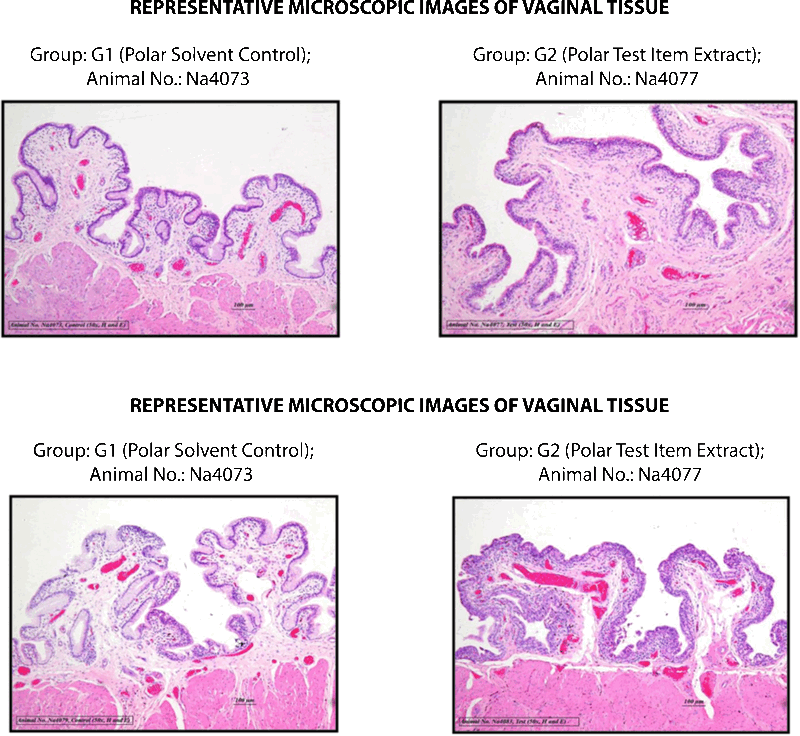
CONCLUSION
Based on the observed results of the experiment and under the experimental conditions employed, the irritation index of both polar and non-polar extracts of Silky Cup (Menstrual Cup) was observed less than one and hence the test item was considered as “non-irritant” to the vagina of New Zealand white rabbit when administered into the vagina for five consecutive days.
STATEMENT OF GLP COMPLIANCE
The Study No.: BIO-TX 2111, entitled “Vaginal Irritation Test of Silky Cup (Menstrual Cup) in New Zealand White Rabbits” was performed in compliance with the OECD Principles of Good Laboratory Practice [C (97)186/Final] and in accordance with FDA Good Laboratory Practice 21 CFR Part 58.
STUDY TITLE: IN VITRO CYTOTOXICITY STUDY OF SILKY CUP (MENSTRUAL CUP) BY ELUTION METHOD
RESULTS AND DISCUSSION
L-929 mouse fibroblast cells of sub confluent monolayer were treated with test item, negative control, positive control and blank in triplicates were incubated for 48 hours at 37°C and 5% CO2. Post treatment with extracts of test item, positive control, negative control and blank, L-929 mouse fibroblast cells were examined microscopically for any changes in cell morphology and cell lysis. L-929 mouse fibroblast cells treated with the test item extract showed discrete intracytoplasmic granules, no cell lysis, no reduction of cell growth (Grade 0). Since there was no reactivity and the grade is not greater than 2, the test item is considered as non-cytotoxic. Cells in the blank wells and negative control showed discrete intracytoplasmic granules, no cell lysis, no reduction of cell growth and no reactivity (Grade 0), whereas the positive control showed nearly complete or complete destruction of the cell layers with severe reactivity (Grade 4).

CONCLUSION
Based on the results obtained under laboratory testing conditions, the test item, Silky Cup (Menstrual Cup) is considered as non-cytotoxic to the subconfluent monolayer of L-929 mouse fibroblast cells.
The Study No. BIO-GT 532, entitled “In vitro Cytotoxicity Study of Silky Cup (Menstrual Cup) by Elution Method” was performed in compliance with the OECD Principles of Good Laboratory Practice [C(97)186/Final] and in accordance with FDA Good Laboratory Practice 21 CFR Part 58.
What is USP Class VI?
The United States Pharmacopoeia (USP) is an independent organisation that established a set of standards to ensure the quality of medicines and health care technologies. Many device manufacturers continue to use the USP Class VI test to determine biocompatibility though ISO 10993 is superseding USP as the reference standard in measuring material biocompatibility. USP protocols are used to classify plastics in Classes I - VI, based on end use, type and time of exposure of human tissue to plastics, of which Class VI requires the most stringent testing of all the six classes.
- Systemic toxicity tests are used to determine the irritant effect of toxic leachables present in extracts of test materials.
- Intracutaneous tests are used to assess the localised reaction of tissue to leachable substances.
- Implantation tests are used to evaluate the reaction of living tissue to the plastic.
STUDY TITLE: USP <88> “CLASS VI” Tests Acute Systemic Toxicity, Intracutaneous Irritation and Intramuscular Implantation.
Systemic Injection
Clinical Observations: None of the test or control animals exhibited overt signs of toxicity at any of the observation points. The test is considered negative because none of the animals injected with extracts of the test article showed a significantly greater biological reaction than the animals treated with the control articles.
Intracutaneous Injection Test
Clinical Observations: There were no overt signs of toxicity observed in any test or control animals. The difference between the test article and control article mean reaction scores (erythema/edema) was less than 1.0. The test article meets the requirements of the Intracutaneous Test.
Implant Test
Clinical Observations: There were no overt signs of toxicity noted in either animal. Macroscopic evaluation of the test and control article implant sites showed no significant infection, encapsulation, hemorrhage, necrosis, or discoloration. The test is considered negative, since in each rabbit the difference between the average scores for all of the categories of biological reaction for the test article and control article implant sites did not exceed 1.0, and the difference between the mean scores for all categories of biological reaction for all of the test article implant sites and the average score for all categories for all the control implant sites did not exceed 1.0. The test article meets the requirements of the Implantation Test.
Conclusion
The USP 0.9% Sodium Chloride for Injection (NaCl), 1 in 20 Ethanol in NaCl (EtOH), and Polyethylene Glycol 400 (PEG) and Cottonseed Oil (CSO) extracts of the test article and the test article, Silky Cup (Menstrual Cup), did not produce a biological response following intramuscular implantation in rabbits, intracutaneous injection in rabbits and systemic injection in mice. Therefore, the test article meets the requirements of the USP guidelines, for Class VI Plastics − 50 °C.
ROHS (Restriction of Hazardous Substances)
Silky Cup is not Non-Toxicity as per Directive 2011/65/EU Restriction of Hazardous Substances Directive (ROHS),
Conclusion
Based on the performed tests on selected part of submitted samples , the result of lead, mercury, cadmium, Hexavalent Chromium,_Polybrominated Biphenyls (PBB)), Polybrominated Diphenylethers (PBDEs) comply with the limits as set by by ROHS Directive 2011/65/EU Annex11: recasting 2002/95/EC
Crack / Flex Test
Repeated bending strain and flexing for crack growth test up to 1,50,000 cycles has been done for Silky Cup
Conclusion
No cut initiation up to 150kcs
